Abstract
Background:
Post-transplant cyclophosphamide (PTCy) has been shown to significantly reduce transplant related mortality (TRM) post hematopoietic stem cell transplantation (HSCT) and is being increasingly used for acute myelogenous leukemia (AML) patients (pts) undergoing HSCT including from unrelated donors (UD). These publications mainly include pts transplanted in first complete remission (CR1). We recently reported outcome in 1879 AML pts transplanted in second CR (CR2) with conventional graft-versus-host disease (GVHD) prophylaxis (Leukemia 2020). Results may differ in transplantation with PTCy as GVHD prophylaxis, eliminating proliferating alloreactive T cells, and upregulating T regulatory cells while suppressing host natural killer (NK) cells immediately after transplantation, changing the biology and characteristics of the transplantation.
Methods: The study aim was to assess outcome of adult AML pts, aged ≥18 years in CR2 undergoing HSCT from a 9-10/10 UD with PTCy, in 2010-2019.Statistics included multivariate analysis (MVA) adjusting for potential confounding factors was performed using a Cox's proportional-hazards regression model for main outcomes.
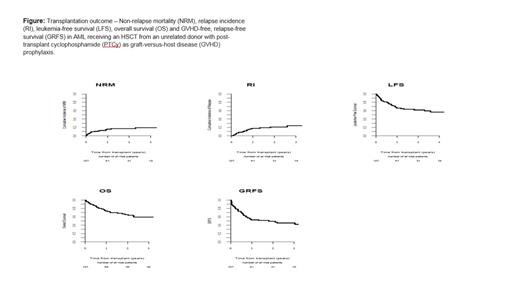
Results: In total, 127 pts were included. Median follow-up was 19.2 (95% CI, 14.7-28) months (mos). Median age was 45.5 (range, 18.2-71.3) years. 54.3% were male. Cytogenetic risk (MRC classification) was favorable, intermediate, and adverse in 15.7%, 55.9%, and 5.5% of pts, respectively (missing data-22.8percentage) Median year of transplantation was 2017. Time from diagnosis to transplantation was 20.4 (range, 4.1-182.4) months. All pts were at CR2 at time of transplantation. Donors were 10/10 and 9/10 UD in 60.6% and 39.4% of pts, respectively. 77.8% and 47.2% of the pts and donors, respectively, were cytomegalovirus (CMV) seropositive. Conditioning was myeloablative in 50.4% and reduced intensity in 49.6%. The most frequent (61.4%) conditioning consisted of busulfan and fludarabine. All pts received PTCy as anti GVHD prophylaxis in combination with immunosuppression, which was cyclosporine A /mycophenolate mofetil (MMF) in 21.3% and MMF/tacrolimus in 23.6%. 33.9% of the pts received In vivo T-cell depletion. Grafts were peripheral blood in 93.7% and bone marrow in 6.3% of transplants. Karnofsky performance score (KPS) was > 90 in 71.2% and the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) was zero in 61.5% of the pts. Engraftment was achieved by 97.6% with day (d) 60 absolute neutrophil count (ANC) > 0.5 x 10 9/L in 96.8%, and d 180 incidence of acute (a) GVHD II-IV and III-IV was 26.2% and 9.2%, respectively. The 2-year total and extensive chronic (c) GVHD was 34.3% and 13.8 %, respectively. The 2-year non-relapse mortality (NRM) was 17.2%. The 2-year relapse incidence (RI) was 21.1%. RI was the main cause of death in 41% of pts who died, followed by infections (23.1%) and GVHD (20.5%). The 2-year leukemia-free survival (LFS), overall survival (OS) and GVHD-free, relapse-free survival (GRFS) was 61.7%, 65.2% and 49.3%, respectively (Figure). In MVA time from diagnosis to transplant was significant prognostic factor for RI, LFS, OS and GRFS hazard ratio (HR) =0.19 (95% CI 0.07-0.48, p<0.001), HR=0.3 (95% CI 0.16-0.56, p<10-3) and HR=0.31 (95% CI 0.15-0.61, p< 0.001), HR=0.40 (95% CI 0.24-0.69, p<0.001), respectively. Year of transplant significantly predict GRFS and aGVHD HR =0.87 (95% CI 0.78-0.97, p<0.009) and HR=0.84 (95% CI 0.73-0.98, p=0.027), respectively. Age was prognostic factor for NRM and good risk cytogenetics for OS HR=1.83 (95% CI 1.24-2.69, p=0.002) and HR=0.21 (95% CI 0.05-0.91, p=0.036), respectively. Finally, female to male combination and RIC were prognostic factors for cGVHD HR=0.15 (95% CI 0.03-0.64, p=0.011), and HR=3.74 (95% CI 1.52-9.18, p=0.004), respectively.
Conclusions: Outcome of AML pts undergoing HSCT from a 9-10/10 MUD in CR with PTCy as GVHD prophylaxis, are similar to previous reports using conventional GVHD prophylaxis with 26% of pts developing aGVHD ,34% cGVHD and NRM of 17%. These results are also similar to those we previously observed in pts undergoing HSCT from UD with PTCy while in CR1.
Labopin: Jazz Pharmaceuticals: Honoraria. Kulagin: X4 Pharmaceuticals, Alexion, Apellis, Biocad: Research Funding; Novartis, Generium, Sanofi, Roche, Johnson & Johnson, Pfizer: Speakers Bureau. Blaise: Jazz Pharmaceuticals: Honoraria. Yakoub-Agha: Jazz Pharmaceuticals: Honoraria. Ciceri: IRCCS Ospedale San Raffaele: Current Employment. Mohty: Sanofi: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Jazz: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Celgene: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal